Discover the pH scale, its range, significance, and real-life applications in chemistry, biology, and health. Learn how acids and bases affect our world.
The pH scale is a crucial scientific concept widely used in chemistry, biology, environmental science, and various industries. It helps measure the acidity or alkalinity of a solution, enabling researchers, students, and professionals to assess chemical reactions, biological functions, water quality, and more.
In this comprehensive article, we will explore:
- What the pH scale is
- Its numerical range
- How it’s measured
- Why it’s essential in science and daily life
- Applications in agriculture, medicine, and environmental science
Table of Contents
What is the pH Scale?
The term pH stands for “potential of hydrogen” or “power of hydrogen.” It measures the concentration of hydrogen ions (H⁺) in a solution. The scale ranges from 0 to 14:
- pH < 7: Acidic
- pH = 7: Neutral
- pH > 7: Alkaline (basic)
pH Formula:
pH=−log10[H+]\text{pH} = -\log_{10}[H^+]pH=−log10[H+]
Where:
- [H⁺] = concentration of hydrogen ions in moles per liter (mol/L)
This logarithmic scale means that each pH unit represents a tenfold change in hydrogen ion concentration.
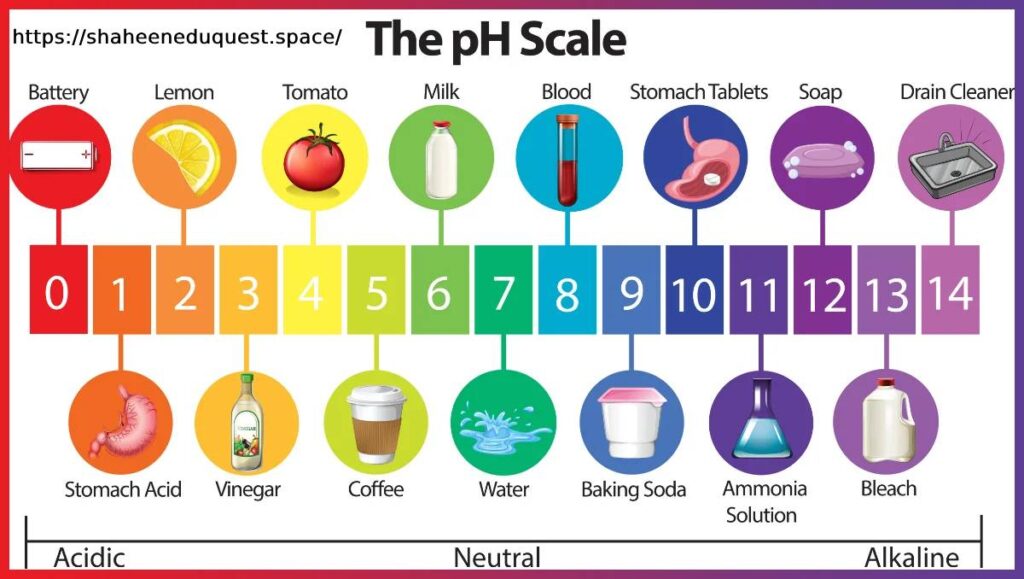
The pH Scale Range Explained
| pH Value | Type | Examples |
|---|---|---|
| 0 – 3 | Strong Acid | Battery acid, hydrochloric acid |
| 4 – 6 | Weak Acid | Vinegar, tomato juice, soft drinks |
| 7 | Neutral | Pure water, human blood (close) |
| 8 – 10 | Weak Base | Baking soda, seawater |
| 11 – 14 | Strong Base | Bleach, lye, ammonia solution |
Fun Fact:
A solution with pH 3 is 10 times more acidic than a solution with pH 4.
How is pH Measured?
pH can be measured using several tools and techniques:
1. pH Indicators
These are substances that change color depending on the acidity or alkalinity of a solution. Common indicators:
- Litmus paper: Turns red in acid, blue in base
- Phenolphthalein: Colorless in acid, pink in base
2. pH Meters
A digital or analog device with an electrode that gives accurate readings. Used in laboratories, agriculture, and industrial settings.
3. Universal Indicator
A mix of indicators providing a color spectrum from red (strong acid) to violet (strong base).
Importance of pH in Everyday Life
In Chemistry
- Determines reaction conditions
- Controls the rate of chemical reactions
- Helps in titrations and buffer solutions
In Agriculture
- Soil pH affects nutrient availability and plant growth
- Ideal soil pH for crops is typically between 6 and 7.5
In Water Quality
- Aquatic life thrives in a specific pH range (6.5 to 8.5)
- Acid rain lowers the pH of lakes and rivers, harming biodiversity
In Human Body
- Blood pH is maintained around 7.35–7.45
- pH imbalance can lead to serious health conditions like acidosis or alkalosis
In Household Products
- Cleaning agents are usually basic
- Skin-care products maintain pH to match skin’s natural acidity (~5.5)
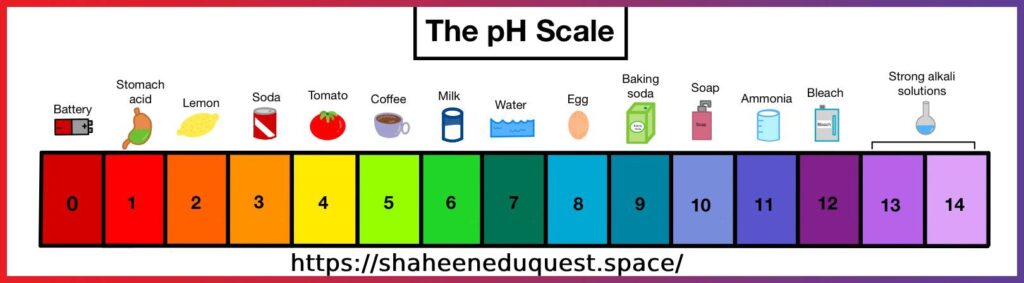
Acids and Bases: A Closer Look
Acids
- Donate hydrogen ions (H⁺)
- Taste sour
- Corrosive in nature
- Turn blue litmus red
Examples: Hydrochloric acid (HCl), sulfuric acid (H₂SO₄), citric acid
Bases
- Accept hydrogen ions or donate hydroxide ions (OH⁻)
- Taste bitter, feel slippery
- Turn red litmus blue
Examples: Sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂)
Neutral Substances
A neutral solution has equal amounts of H⁺ and OH⁻ ions. Its pH is exactly 7.
Examples:
- Pure water
- Saline solution
- Milk (close to neutral)
What Affects pH?
Several factors can change the pH of a solution:
- Concentration of solutes
- Temperature (affects ionization)
- Chemical reactions
- Addition of acids or bases
Buffer Solutions: Maintaining pH Balance
Buffer solutions resist changes in pH when small amounts of acids or bases are added. They are critical in:
- Maintaining blood pH
- Controlling pH in fermentation and pharmaceuticals
- Stabilizing pH in chemical experiments
Example: Bicarbonate buffer in the human body
pH in Biology and Medicine
Human Body
- Enzymes work best at specific pH values
- The stomach’s pH (1.5–3.5) helps digest food
- Blood has a buffering system to keep pH stable
Medical Applications
- Urine tests for pH help diagnose kidney conditions
- pH affects drug solubility and absorption
pH and the Environment
Soil pH
- Influences microbial activity and root development
- Acidic soil may require lime (CaCO₃) to neutralize pH
Acid Rain
- Forms when sulfur dioxide (SO₂) and nitrogen oxides (NOx) dissolve in rainwater
- Damages buildings, crops, and aquatic systems
Aquatic Life
- Fish and aquatic plants need a balanced pH
- Low pH harms gill function and reproduction
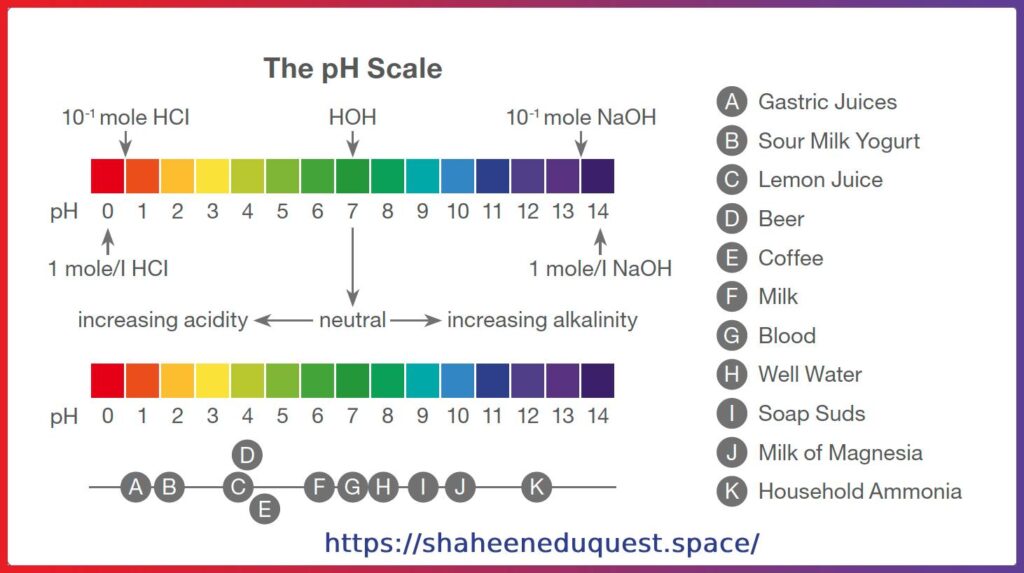
Industrial Applications of pH
- Food Industry: Controls flavor and shelf life (e.g., vinegar, pickles)
- Beverage Industry: pH influences fermentation
- Pharmaceuticals: Drug formulation and stability
- Cosmetics: Ensures products are skin-safe
- Textile Industry: Dye fixation requires pH control
pH Scale and Logarithmic Nature
Understanding the logarithmic nature of the pH scale is vital. It means:
- A solution with pH 2 is 100 times more acidic than pH 4
- Every 1 unit change equals a tenfold increase or decrease in H⁺ concentration
This sensitivity highlights the need for precise measurements, especially in scientific fields.
Real-World Examples and Case Studies
Case Study 1: Swimming Pools
Maintaining a pH between 7.2 and 7.8 is crucial to prevent irritation and equipment damage.
Case Study 2: Crop Yield and Soil pH
Farmers test soil pH to ensure maximum nutrient availability. For example, wheat grows best at a pH of around 6.5.
Case Study 3: Wastewater Treatment
Plants use pH adjustment to neutralize acidic or basic industrial waste before releasing it into the environment.
How to Adjust pH
To Increase pH (More Basic):
- Add substances like baking soda (NaHCO₃) or lime (CaO)
To Decrease pH (More Acidic):
- Add citric acid, vinegar (acetic acid), or sulfuric acid in controlled amounts
Safety and Handling of Acids and Bases
Always follow safety precautions:
- Wear gloves, goggles, and lab coats
- Handle strong acids/bases in well-ventilated areas
- Never mix chemicals without guidance
- Store pH-altering substances safely and label clearly
Conclusion
The pH scale is a foundational concept in science that touches nearly every aspect of our lives—from the health of our bodies and the food we eat to environmental sustainability and industrial manufacturing. Understanding and managing pH not only enhances scientific knowledge but also empowers better decision-making in real-world situations.
By recognizing the importance of pH, we unlock the ability to monitor, adjust, and optimize countless processes in chemistry, biology, medicine, agriculture, and environmental science.
