Understanding atoms and molecules isn’t just for scientists in white coats. It’s essential knowledge that explains how the world works, from the fizz in a soda to the functioning of your smartphone battery.here we’ll break down the world of atoms and molecules in a way that’s easy to understand yet detailed enough to satisfy your curiosity.
Table of Contents
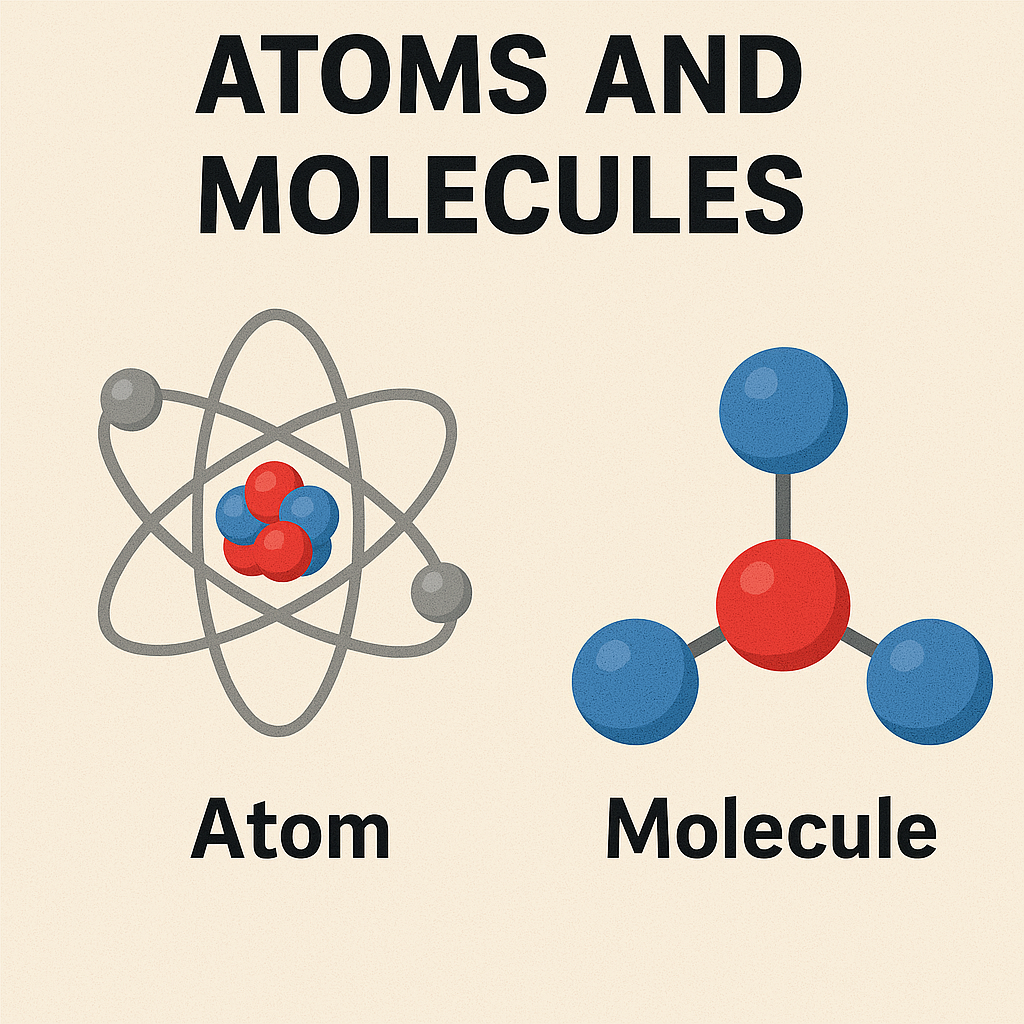
The Concept of Atoms
The term “atom” comes from the Greek word atomos, meaning indivisible. Ancient philosophers like Democritus theorized that everything in the universe was made up of tiny, indivisible particles. Although this idea was purely philosophical back then, modern science has confirmed that atoms do exist—though they aren’t exactly indivisible.
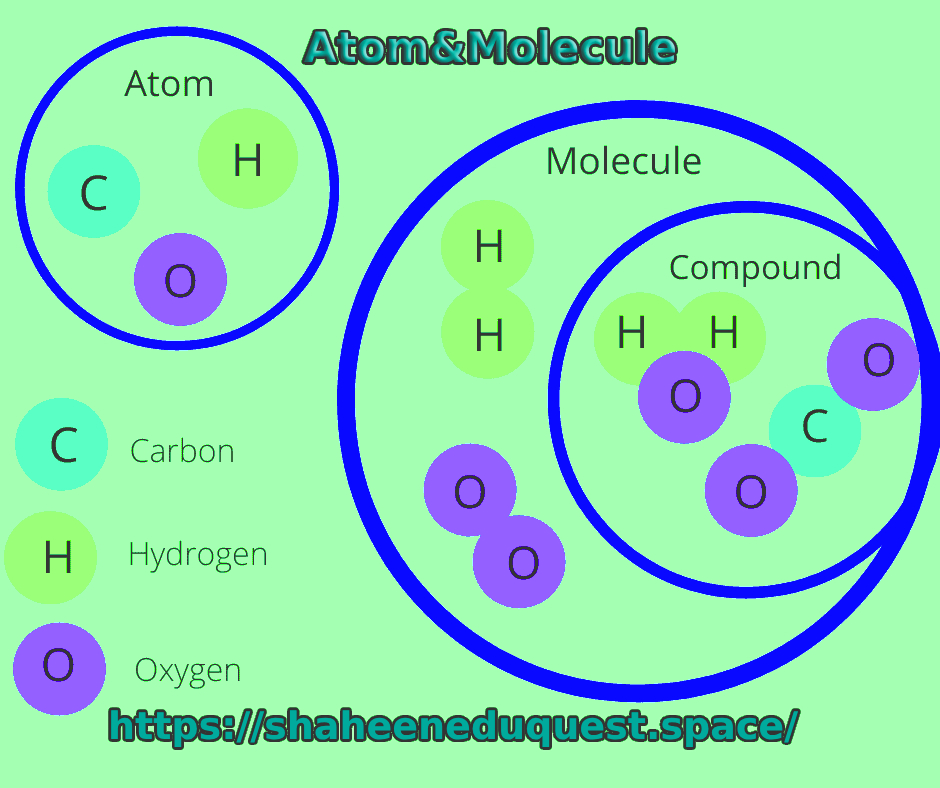
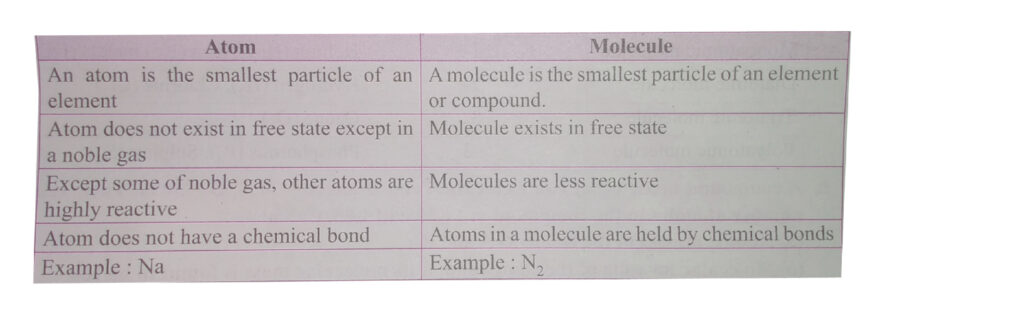
An atom is the smallest unit of an element that retains the chemical properties of that element. For example, a single atom of oxygen still behaves like oxygen. But if you split it any further, it no longer retains its elemental identity.
Each atom is composed of three main subatomic particles: protons, neutrons, and electrons. Protons and neutrons are found in the atom’s central core, known as the nucleus. Electrons, on the other hand, orbit around the nucleus in regions known as electron shells or energy levels.
Subatomic Particles at a Glance
- Protons are positively charged particles found in the nucleus. The number of protons determines the atomic number, which defines the element.
- Neutrons are neutral particles (no charge) also located in the nucleus. They contribute to the atom’s mass and play a role in stability.
- Electrons are negatively charged particles that orbit the nucleus. Their arrangement influences chemical behavior and bonding.
Atomic Structure and Identity
Each element in the periodic table is defined by its atomic number, which is the number of protons in its atoms. For example, hydrogen has one proton, so its atomic number is 1. Carbon has six protons, so its atomic number is 6. This number is critical because changing the number of protons changes the element entirely.
Electrons usually equal the number of protons in a neutral atom, which balances out the electrical charge. Neutrons can vary in number even within atoms of the same element. These variations are known as isotopes. For instance, carbon-12 and carbon-14 are both isotopes of carbon.
What Are Molecules?
While atoms are the basic units of elements, molecules are the basic units of compounds. A molecule forms when two or more atoms chemically bond together. The atoms in a molecule can be of the same element—like O₂ (oxygen gas), which consists of two oxygen atoms—or of different elements, like H₂O (water), made of two hydrogen atoms and one oxygen atom.
Molecules can be simple or complex. Simple molecules include diatomic elements like nitrogen (N₂) or compounds like carbon dioxide (CO₂). Complex molecules, such as proteins or DNA, may consist of thousands of atoms bonded in intricate arrangements.
Chemical Bonds and Molecular Formation
Atoms don’t just randomly stick together. Their ability to bond depends on their electron configurations, particularly the electrons in the outermost shell, called valence electrons.
There are three main types of chemical bonds that lead to molecule formation:
1. Covalent Bonds
In covalent bonding, atoms share one or more pairs of electrons. This type of bond is common in organic compounds and molecules like water (H₂O), methane (CH₄), and oxygen (O₂). Covalent bonds are usually strong and stable.
2. Ionic Bonds
Ionic bonds occur when one atom donates an electron to another, resulting in oppositely charged ions that attract each other. A classic example is sodium chloride (NaCl), or table salt. Sodium (Na) donates an electron to chlorine (Cl), forming positively charged sodium ions and negatively charged chloride ions.
3. Metallic Bonds
These occur between metal atoms, where electrons are shared in a sort of “sea of electrons” that flows freely around the nuclei. This unique arrangement gives metals their characteristic properties like conductivity and malleability.
Molecules vs. Compounds
It’s important to note the difference between molecules and compounds. All compounds are molecules, but not all molecules are compounds. A molecule is a group of two or more atoms bonded together. If those atoms are of different elements, it’s a compound.
For example:
- O₂ is a molecule (not a compound, because it’s made of the same element).
- H₂O is both a molecule and a compound.
The Importance of Molecular Structure
The arrangement of atoms within a molecule—its molecular structure—can drastically impact its properties. Consider carbon, for example. In one form, it makes up the soft, black substance known as graphite. In another, it becomes the hardest substance on Earth: diamond. Both are made entirely of carbon atoms but arranged in very different structures.
The shape of a molecule affects its behavior, interactions, polarity, and how it reacts with other substances. Molecular geometry is a huge area of study in chemistry and is essential in fields like pharmacology and materials science.
States of Matter and Molecular Behavior
Atoms and molecules behave differently depending on the state of matter: solid, liquid, gas, or plasma.
- In solids, atoms and molecules are tightly packed and vibrate in fixed positions. This structure gives solids a definite shape and volume.
- In liquids, molecules are close together but can move around, allowing the liquid to flow and take the shape of its container.
- In gases, molecules are far apart and move freely, which is why gases expand to fill their container.
- In plasma, atoms are ionized, meaning they have lost or gained electrons, and exist in a high-energy state. Plasmas are found in stars, including our sun.
The Mole Concept
In chemistry, it’s often impractical to count individual atoms or molecules because they are so tiny and numerous. Instead, scientists use the mole concept. One mole of any substance contains 6.022 × 10²³ particles (Avogadro’s number). This concept allows chemists to work with substances on a manageable scale.
For instance, one mole of water molecules (H₂O) contains 6.022 × 10²³ water molecules, and it weighs about 18 grams.
Atoms in Chemical Reactions
During chemical reactions, atoms are rearranged to form new molecules. The atoms themselves are not created or destroyed, which aligns with the law of conservation of mass. Instead, chemical bonds are broken and new ones are formed.
An example of a chemical reaction is the formation of water:
2H₂ + O₂ → 2H₂O
Here, hydrogen and oxygen gases react to form water. Notice that the number of atoms on each side remains the same—just reorganized.
The Role of Atoms and Molecules in Everyday Life
Atoms and molecules may be microscopic, but their impact on our lives is anything but small. Here are just a few ways they influence our daily existence:
- Food and digestion: Enzymes, proteins, and carbohydrates are all made of molecules interacting at the atomic level.
- Breathing: Oxygen molecules (O₂) are inhaled and used by cells to produce energy.
- Medicines: Drugs are carefully designed molecules that interact with biological molecules in the body.
- Technology: Semiconductors and batteries operate based on the controlled behavior of atoms and molecules.
- Cleaning products: Soaps and detergents work because of molecular structures that can attract both water and oil.
Modern Applications and Research
In recent decades, the study of atoms and molecules has exploded into new and exciting territories.
Nanotechnology
Nanotechnology manipulates atoms and molecules to create materials and devices at the nanoscale—just billionths of a meter wide. This technology holds promise for medicine, electronics, energy, and more.
Molecular Biology
This field studies the molecular basis of life. DNA, RNA, proteins—all are molecules with specific atomic arrangements. Understanding them helps scientists cure diseases, improve crops, and even edit genes.
Quantum Chemistry
Quantum chemistry explores how quantum mechanics influences the behavior of electrons in atoms and molecules. This field has given rise to better models for predicting chemical behavior and has improved everything from drug design to materials science.
Climate Science
Molecules like CO₂ (carbon dioxide), CH₄ (methane), and H₂O (water vapor) are greenhouse gases that trap heat in Earth’s atmosphere. Understanding their molecular behavior is critical to studying and combating climate change.
Atoms and Molecules in Space
Even in the vast emptiness of space, atoms and molecules play a crucial role. Interstellar clouds, made of gas and dust, contain hydrogen atoms and simple molecules. These clouds can collapse under gravity to form stars and planets. Astronomers even study the spectral fingerprints of molecules in distant galaxies to understand cosmic events and the potential for extraterrestrial life.
The Beauty of the Invisible World
Though we can’t see atoms and molecules with our eyes, the beauty of their world is breathtaking. With the help of powerful electron microscopes and computer models, scientists can now visualize molecular structures. The symmetry, complexity, and elegance of these structures often resemble intricate works of art—only they form the essence of reality.
From the water in our bodies to the stars in the sky, atoms and molecules are the unifying thread of existence. Their study isn’t just science—it’s a quest to understand the universe at its most fundamental level.
Conclusion
Atoms and molecules may be tiny, but they are mighty. They’re not just scientific abstractions; they are the very stuff of life. Every breath we take, every bite of food we eat, every object we touch—all are governed by the intricate dance of atoms and molecules.
By learning about atoms and molecules, we begin to see the world differently. We start to appreciate the deep connections between chemistry, biology, physics, and even the cosmos. And perhaps most importantly, we gain a better understanding of our place in this vast and interconnected universe.