Explore the complete difference between atoms and molecules with definitions, characteristics, examples, and comparisons. Ideal for students and science enthusiasts.
Understanding the basic building blocks of matter is essential for studying chemistry and physics. Two of the most fundamental terms in science are atoms and molecules. While these terms are often used interchangeably in casual conversation, they have distinct meanings in the scientific world. In this in-depth guide, we’ll explore what atoms and molecules are, how they differ, and why understanding the distinction is crucial.
Table of Contents
- Introduction
- What is an Atom?
- What is a Molecule?
- Key Differences Between Atoms and Molecules
- Types of Molecules
- Importance in Chemistry and Physics
- Real-Life Examples
- The Role of Atoms and Molecules in Biological Systems
- Frequently Asked Questions
- Conclusion
Atoms and molecules form the core of scientific studies related to matter. All substances, whether solid, liquid, or gas, are composed of these tiny particles. Although invisible to the naked eye, they dictate the structure, properties, and behavior of all materials around us.
Understanding the difference between atoms and molecules is not only fundamental to chemistry but also plays a vital role in physics, biology, and materials science.
2. What is an Atom?
Definition
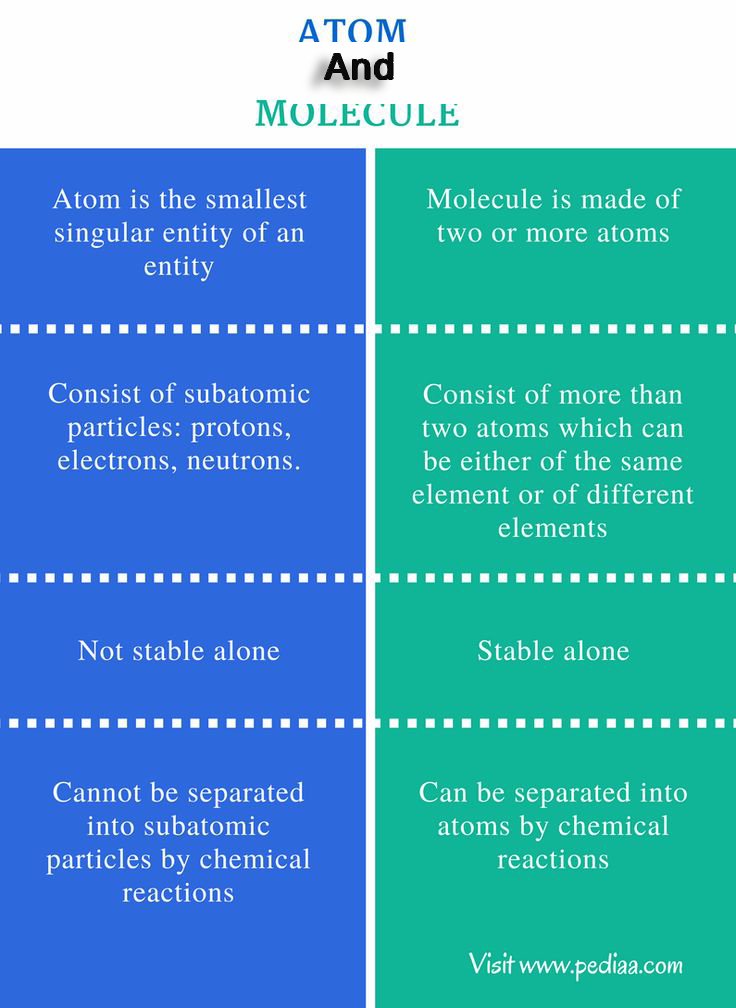
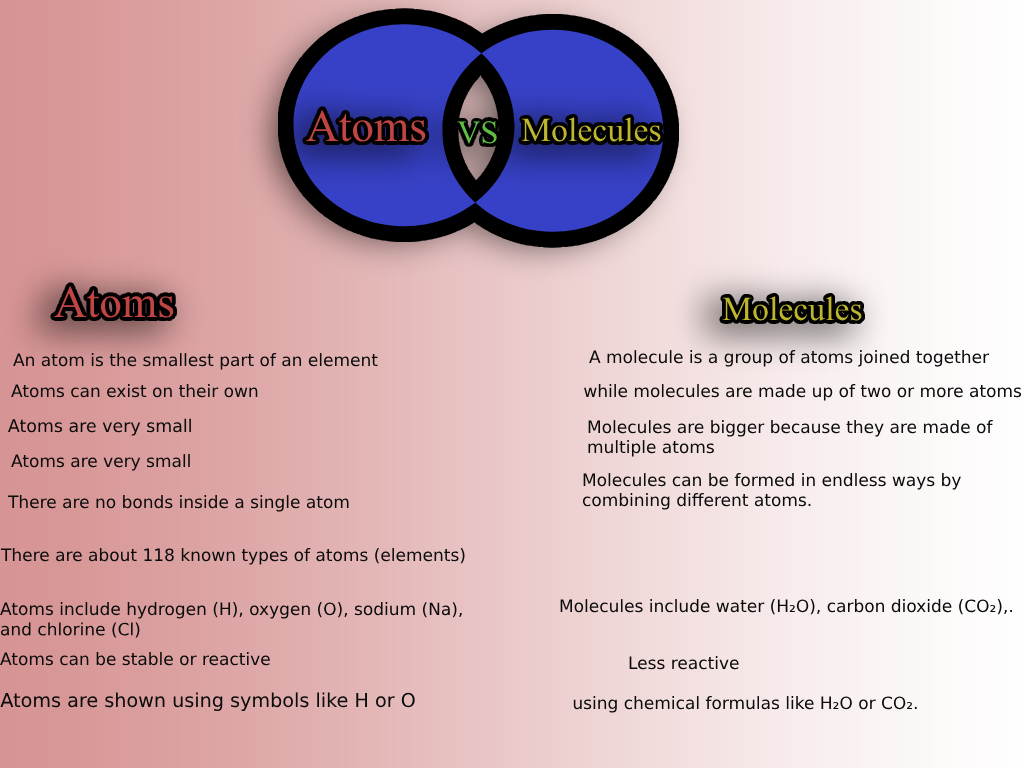
An atom is the smallest unit of an element that retains the properties of that element. It consists of a nucleus containing protons and neutrons, surrounded by a cloud of electrons.
Components of an Atom
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles that also reside in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells.
Characteristics of Atoms
- Atoms are electrically neutral (equal number of protons and electrons).
- Each element in the periodic table is composed of unique atoms.
- Atoms are extremely small—typically about 0.1 nanometers in size.
- The atomic number (number of protons) defines the element.
- Atoms can exist independently or combine with other atoms to form molecules.
Examples of Atoms
- Hydrogen (H)
- Oxygen (O)
- Carbon (C)
- Iron (Fe)
- Gold (Au)
3. What is a Molecule?
Definition
A molecule is a group of two or more atoms that are chemically bonded together. Molecules can consist of the same type of atoms (elemental molecules) or different types of atoms (compound molecules).
Characteristics of Molecules
- Molecules are formed via chemical bonds, such as covalent bonds or ionic bonds.
- Molecules can exist as diatomic, triatomic, or polyatomic structures.
- Molecules have a definite structure and composition.
- They can be broken down into atoms or smaller molecules through chemical reactions.
Examples of Molecules
- O₂ – Molecular oxygen (diatomic molecule)
- H₂O – Water (compound molecule)
- CO₂ – Carbon dioxide
- CH₄ – Methane
- C₆H₁₂O₆ – Glucose
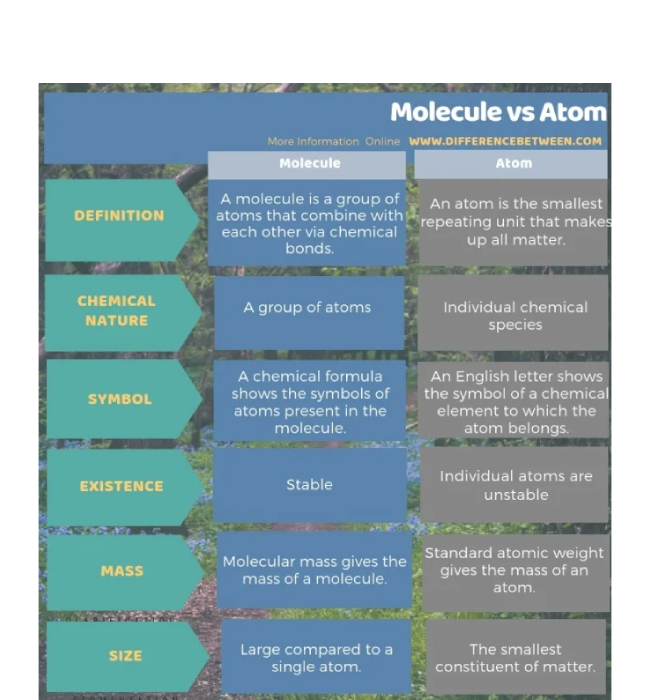
4. Key Differences Between Atoms and Molecules
Here is a detailed comparison to clearly differentiate between atoms and molecules:
| Feature | Atom | Molecule |
|---|---|---|
| Definition | Smallest unit of an element | Group of atoms chemically bonded |
| Existence | Can exist independently | Made up of two or more atoms |
| Size | Smaller | Larger than atoms |
| Bonding | No bonds within a single atom | Chemical bonds hold atoms together |
| Types | 118 known elements | Infinite varieties depending on atomic combinations |
| Examples | H, O, Na, Cl | H₂O, CO₂, NH₃ |
| Stability | Can be reactive or stable | Often more stable than individual atoms |
| Representation | Represented by atomic symbols (H, O) | Represented by chemical formulas (H₂O, CO₂) |
5. Types of Molecules
Molecules can be categorized based on the number and types of atoms involved.
A. Elemental Molecules
Composed of two or more same type of atoms.
- Examples: O₂, N₂, H₂, Cl₂
B. Compound Molecules
Formed from different atoms bonded together.
- Examples: H₂O (water), CO₂ (carbon dioxide), CH₄ (methane)
C. Organic Molecules
Contain carbon and are found in living organisms.
- Examples: C₆H₁₂O₆ (glucose), C₂H₆O (ethanol)
D. Inorganic Molecules
Do not contain carbon-hydrogen bonds.
- Examples: H₂O, NaCl
6. Importance in Chemistry and Physics
Atoms in Chemistry
- The Periodic Table organizes atoms based on atomic number.
- Chemical reactions involve the rearrangement of atoms.
- Isotopes are atoms of the same element with different neutron numbers.
Molecules in Chemistry
- Molecules are central to the study of chemical reactions.
- Molecular structure determines physical and chemical properties.
- Molecular formulas and structural formulas represent chemical compounds.
In Physics
- Atomic theory explains the nature of matter and its interactions.
- Quantum physics explores electron behavior in atoms.
- Molecular physics studies vibration and rotation of molecules.
7. Real-Life Examples
Water (H₂O)
- A molecule made of two hydrogen atoms and one oxygen atom.
- Atoms individually are gases; combined, they form liquid water.
Oxygen (O₂)
- Two oxygen atoms form a stable molecule essential for respiration.
Table Salt (NaCl)
- Made of sodium (Na) and chlorine (Cl) atoms in a 1:1 ratio.
- Ionic bonding forms a crystalline molecule.
Glucose (C₆H₁₂O₆)
- Organic molecule crucial for metabolism in living organisms.
8. The Role of Atoms and Molecules in Biological Systems
Atoms and molecules are not just abstract scientific concepts—they are essential to life.
DNA
- DNA molecules carry genetic information.
- Composed of atoms of carbon, hydrogen, nitrogen, oxygen, and phosphorus.
Proteins
- Long chains of amino acid molecules.
- Composed of atoms like C, H, N, O, and S.
Enzymes
- Molecules that catalyze biological reactions.
- Specific molecular structures allow for target interactions.
Metabolism
- Involves chemical reactions between atoms and molecules in the body.
- Example: Glucose reacts with oxygen to release energy.
9. Frequently Asked Questions
Q1: Can an atom be a molecule?
Not exactly. An atom is a single particle, while a molecule is a group of atoms. However, some elements exist naturally as molecules (e.g., O₂), so their atoms are part of molecules in nature.
Q2: Are molecules always visible?
No. Molecules are incredibly small and can only be observed with powerful microscopes like the scanning tunneling microscope (STM) or inferred through spectroscopy.
Q3: What keeps atoms together in a molecule?
Atoms are held together in a molecule by chemical bonds, primarily covalent bonds (sharing electrons) or ionic bonds (transfer of electrons).
Q4: How do atoms combine to form molecules?
Through chemical bonding, atoms achieve a stable electron configuration. This results in the formation of molecules with lower potential energy.
Q5: Why is it important to distinguish atoms from molecules?
Understanding this distinction is essential for learning about:
- Chemical reactions
- Stoichiometry
- Molecular biology
- Material properties
10. Conclusion
The fundamental difference between atoms and molecules lies in their structure and function. Atoms are the simplest units of matter, each representing a single chemical element. Molecules, on the other hand, are combinations of two or more atoms bonded together, forming the basis for chemical compounds.
Whether you’re exploring the composition of air, analyzing water, or studying organic life forms, atoms and molecules are at the core of your understanding. Differentiating them is crucial not just for academic knowledge, but also for real-world applications in medicine, engineering, materials science, and environmental studies.