Learn the key differences between reversible and irreversible reactions with definitions, examples, comparison table, and real-life applications. Understand their roles in chemistry and industry Understanding the distinction between these two types of reactions is essential for anyone delving into the fields of chemistry, biochemistry, chemical engineering, or environmental science. This article delves deep into the definitions, mechanisms, characteristics, differences, and real-world applications of reversible and irreversible reactions.
Table of Contents
What is a Chemical Reaction?
Before diving into the specifics, it’s essential to understand what a chemical reaction is. A chemical reaction involves the transformation of reactants into products through the breaking and forming of chemical bonds. These reactions can proceed in one direction or both directions, leading to the classification into Reversible and Irreversible Reactions
What is a Reversible Reaction?
A reversible reaction is a chemical reaction where the conversion of reactants to products and the conversion of products back to reactants happen simultaneously.
General Representation:
A + B ⇌ C + D
Here, the double arrow (⇌) indicates that the reaction can proceed in both directions—forward and backward—until it reaches a state of equilibrium.
Characteristics of Reversible Reactions:
- Dynamic Equilibrium: The forward and reverse reactions occur at the same rate at equilibrium.
- Partial Conversion: Reactants are never fully converted into products.
- Affected by Conditions: Temperature, pressure, and concentration can shift the reaction equilibrium.
- No Net Change at Equilibrium: Although the reactions are ongoing, the concentrations of reactants and products remain constant.
Examples of Reversible Reactions:
- Haber Process:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g) - Esterification Reaction:
CH₃COOH + C₂H₅OH ⇌ CH₃COOC₂H₅ + H₂O - Carbonic Acid Formation in Blood:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻

What is an Irreversible Reaction?
An irreversible reaction is a chemical reaction that proceeds in one direction only—reactants are completely converted into products, and the reverse process does not occur under the same conditions.
General Representation:
A + B → C + D
Here, the single arrow (→) indicates the one-way direction of the reaction.
Characteristics of Irreversible Reactions:
- Complete Conversion: Reactants are fully converted into products.
- No Equilibrium State: Reaction goes to completion without reaching equilibrium.
- Product Removal: Products usually leave the system or are not reactive under the same conditions.
- Often Fast and Exothermic: Many irreversible reactions release a lot of energy.
Examples of Irreversible Reactions:
- Combustion Reaction:
CH₄ + 2O₂ → CO₂ + 2H₂O - Neutralization Reaction:
HCl + NaOH → NaCl + H₂O - Rusting of Iron:
4Fe + 3O₂ → 2Fe₂O₃
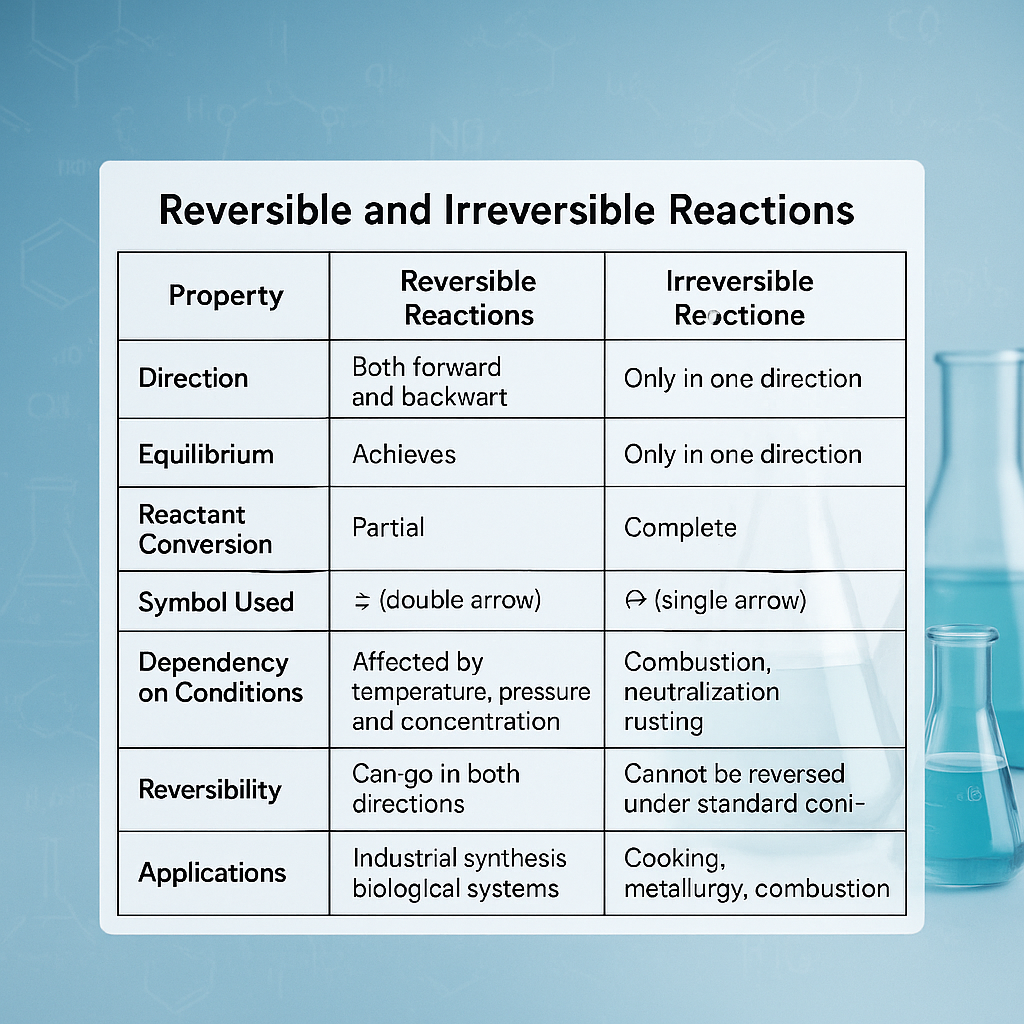
Comparison Table: Reversible vs Irreversible Reactions
| Property | Reversible Reactions | Irreversible Reactions |
|---|---|---|
| Direction | Both forward and backward | Only in one direction |
| Equilibrium | Achieves dynamic equilibrium | No equilibrium established |
| Reactant Conversion | Partial | Complete |
| Symbol Used | ⇌ (double arrow) | → (single arrow) |
| Dependency on Conditions | Affected by temperature, pressure, and concentration | Not significantly affected once started |
| Examples | Haber process, esterification, carbonic acid in blood | Combustion, neutralization, rusting |
| Reversibility | Can go in both directions | Cannot be reversed under standard conditions |
| Applications | Industrial synthesis, biological systems | Cooking, metallurgy, combustion |
Understanding Chemical Equilibrium in Reversible Reactions
A key concept in reversible reactions is chemical equilibrium, a dynamic state where the rate of the forward reaction equals the rate of the reverse reaction. At this point, the concentrations of reactants and products remain unchanged.
Le Chatelier’s Principle:
This principle states that if an external condition such as concentration, temperature, or pressure is changed, the system will adjust itself to counteract the effect of the change and restore a new equilibrium.
Example: In the Haber Process: N₂ + 3H₂ ⇌ 2NH₃
- Increasing pressure favors ammonia formation.
- Increasing temperature favors the reverse reaction (endothermic direction).
Energy Profile Diagrams&Reversible and Irreversible Reactions
Reversible Reaction:
The energy diagram shows two activation energy peaks, one for the forward reaction and one for the reverse. The reaction can go in both directions depending on the energy input.
Irreversible Reaction:
The energy diagram has a steep downward curve, representing the exothermic release of energy. The reverse reaction is not feasible under standard conditions.

Real-Life Applications
Reversible Reactions:
- Industrial Processes:
- Haber Process for ammonia production.
- Contact Process for sulfuric acid.
- Physiological Processes:
- Oxygen and carbon dioxide exchange in the lungs and tissues.
- Buffer systems in blood to maintain pH.
- Environmental Systems:
- Carbonate buffering in oceans helps regulate atmospheric CO₂.
Irreversible Reactions:
- Combustion:
- Fuels like gasoline undergo irreversible combustion to power engines.
- Cooking and Baking:
- Cooking eggs or baking bread involves irreversible protein denaturation.
- Metallurgy:
- Extraction of metals from ores involves irreversible redox reactions.
Factors Affecting Reversible and Irreversible Reactions
For Reversible Reactions:
- Temperature: Can favor forward or reverse depending on reaction enthalpy.
- Pressure: Affects gaseous reactions (especially those involving volume change).
- Concentration: Altering reactant/product concentrations can shift equilibrium.
For Irreversible Reactions:
- Catalysts: Speed up the reaction but don’t affect direction.
- Energy Released: Often highly exothermic, discouraging reverse reaction.
- Solubility of Products: Precipitates or gases leaving the system make the reverse process unfeasible.
Importance in Chemical Industry
The optimization of reversible reactions is critical in maximizing yields and efficiency. By manipulating external conditions (as per Le Chatelier’s Principle), chemists can direct equilibrium to favor product formation.
In contrast, irreversible reactions are utilized for permanent transformations—for example, in fuel burning or synthetic material production—where product permanence is desired.
Examples from Biochemistry
Reversible Reactions in the Body:
- Enzyme reactions: Many enzymes catalyze reversible reactions allowing metabolic flexibility.
- ATP ↔ ADP + Pi: The hydrolysis and synthesis of ATP is a reversible energy transaction.
Irreversible Reactions in the Body:
- Glycolysis Step 1: Glucose → Glucose-6-phosphate (traps glucose in the cell).
- Blood clotting: A cascade of irreversible enzyme activations.
FAQs on Reversible and Irreversible Reactions
Q1. Can an irreversible reaction become reversible?
A: Some reactions are theoretically reversible, but under standard lab conditions, they act as irreversible due to product removal or high energy barriers.
Q2. How do catalysts influence reversibility?
A: Catalysts speed up both forward and reverse reactions equally but do not affect the equilibrium position or reversibility.
Q3. Is photosynthesis reversible?
A: The net photosynthesis process (CO₂ + H₂O → C₆H₁₂O₆ + O₂) is practically irreversible under natural conditions due to energy input from sunlight.
Q4. Are all combustion reactions irreversible?
A: Yes, under normal conditions, combustion is irreversible because the energy required to reform the original substances is prohibitively high.
Conclusion about Reversible and Irreversible Reactions
The distinction between reversible and irreversible reactions is crucial in understanding chemical behavior, controlling industrial processes, and interpreting biological functions. Reversible reactions, governed by equilibrium and dynamic exchange, offer flexibility and control. Irreversible reactions, characterized by complete transformation, are fundamental in energy production and material processing.
Whether it’s the balanced workings of the human body or the controlled environments of industrial synthesis, both types of reactions showcase the beautiful complexity and efficiency of chemistry. By mastering these concepts, learners and professionals can appreciate the delicate interplay of conditions, kinetics, and energy that drive the world around us.