Stoichiometry is a central concept in chemistry that enables scientists to quantify the relationships between reactants and products in chemical reactions. Derived from the Greek words “stoicheion” (meaning element) and “metron” (meaning measure), stoichiometry provides a mathematical framework to understand how matter is conserved and transformed during chemical changes.
This article explores the fundamentals of stoichiometry, its importance in chemistry, key laws, types, and real-world applications. Whether you’re a student, teacher, or enthusiast, this comprehensive guide will deepen your understanding of one of chemistry’s most essential tools.
Table of Contents
What is Stoichiometry?
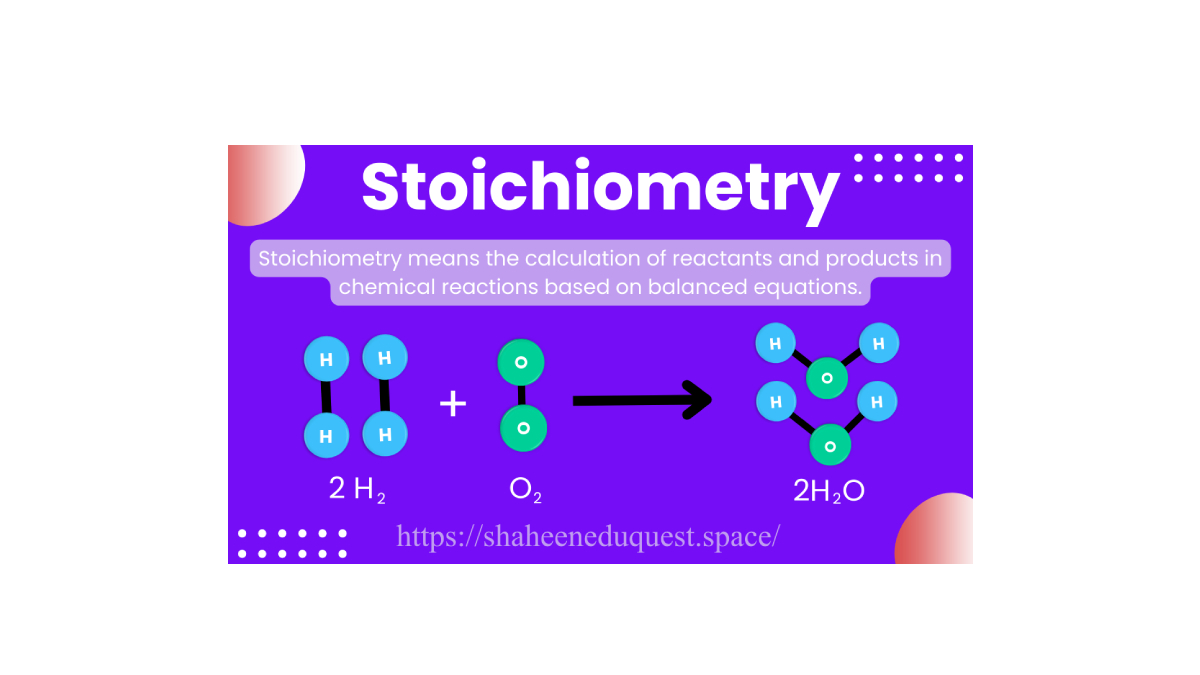
Stoichiometry is the quantitative study of reactants and products in a chemical reaction. It involves calculating the amounts (in moles, grams, or volume) of substances involved in a reaction based on a balanced chemical equation.
For example, consider the reaction:
2H₂ + O₂ → 2H₂O
This balanced equation tells us that 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water. Stoichiometry allows us to calculate how much hydrogen is needed to produce a certain amount of water, or how much oxygen will be left unreacted.
Importance of Stoichiometry in Chemistry
Stoichiometry is a foundational concept for several reasons:
- Quantifies Chemical Reactions
It helps predict how much product can be formed from a given amount of reactants. - Conservation of Mass
Supports the Law of Conservation of Mass, which states that mass is neither created nor destroyed in a chemical reaction. - Industrial Applications
Used in manufacturing industries to maximize yield and minimize waste of raw materials. - Chemical Engineering & Pharmacology
Plays a crucial role in drug formulation, energy generation, and environmental control systems.
Laws Behind Stoichiometry
Stoichiometry is governed by fundamental laws of chemistry:
1. Law of Conservation of Mass
This law, proposed by Antoine Lavoisier, states that mass is conserved in every chemical reaction. The total mass of reactants equals the total mass of products.
2. Law of Definite Proportions
Proposed by Joseph Proust, this law asserts that a given compound always contains the same elements in the same ratio by mass.
3. Law of Multiple Proportions
Proposed by John Dalton, this law states that when two elements combine to form more than one compound, the ratios of the masses of the second element that combine with a fixed mass of the first element are in whole numbers.
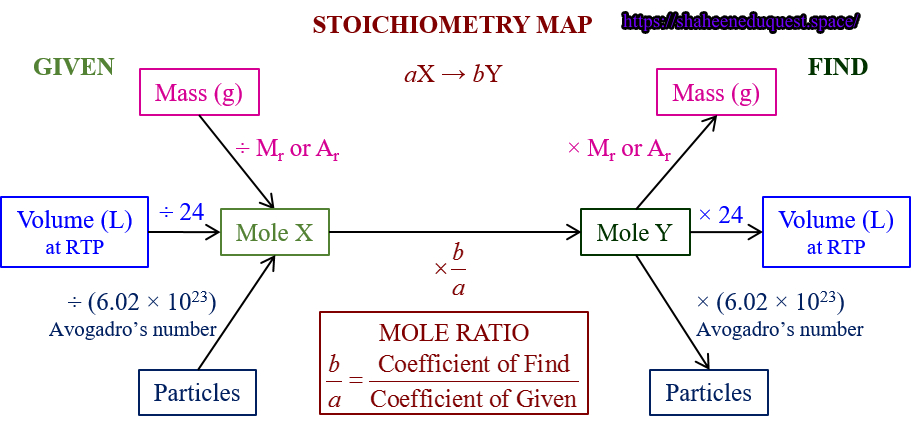
Types of Stoichiometric Calculations
Stoichiometry calculations are categorized based on the data and requirement:
1. Mole-to-Mole Conversions
Determine the amount of one substance in moles from the known amount of another substance in moles.
Example: If 3 moles of H₂ are used, how many moles of H₂O will form? From the equation: 2H₂ + O₂ → 2H₂O,
3 mol H₂ × (2 mol H₂O / 2 mol H₂) = 3 mol H₂O
2. Mass-to-Mass Conversions
Convert grams of a reactant to grams of a product using molar masses and mole ratios.
Example: How many grams of water are formed from 4 grams of hydrogen?
- Convert grams of H₂ to moles.
- Use mole ratio to find moles of H₂O.
- Convert moles of H₂O to grams.
3. Limiting Reactant Problems
The limiting reactant is the substance that gets consumed first, limiting the amount of product formed.
4. Percent Yield
Represents the efficiency of a reaction: Percent Yield = (Actual Yield / Theoretical Yield) × 100
Step-by-Step Guide to Stoichiometric Calculations
Let’s walk through a standard stoichiometry problem:
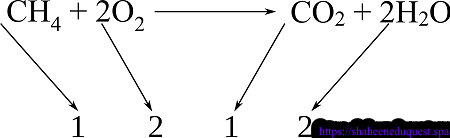
Question: How many grams of CO₂ are produced when 10 g of CH₄ burns in excess oxygen?
Balanced Reaction: CH₄ + 2O₂ → CO₂ + 2H₂O
Step 1: Convert grams of CH₄ to moles
Molar mass of CH₄ = 16 g/mol
10 g CH₄ ÷ 16 g/mol = 0.625 mol CH₄
Step 2: Use mole ratio from balanced equation
1 mol CH₄ → 1 mol CO₂
So, 0.625 mol CH₄ → 0.625 mol CO₂
Step 3: Convert moles of CO₂ to grams
Molar mass of CO₂ = 44 g/mol
0.625 mol CO₂ × 44 g/mol = 27.5 g CO₂
Answer: 27.5 grams of CO₂ are produced.
Real-Life Applications of Stoichiometry
1. Pharmaceuticals
Drug manufacturing requires precise stoichiometric calculations to ensure correct dosage and avoid harmful byproducts.
2. Food Industry
Stoichiometry is used in fermentation processes and food preservation to balance chemical reactions.
3. Environmental Chemistry
Helps in calculating pollutants, optimizing reactions to reduce harmful emissions.
4. Agriculture
Fertilizer production and soil nutrient balancing depend on stoichiometric principles.
5. Energy Sector
In fuel combustion, stoichiometry helps determine fuel requirements for engines and power plants.
Green Chemistry & Stoichiometry
Modern chemistry emphasizes sustainability. Stoichiometry helps in:
- Designing atom-efficient reactions (maximizing atom economy)
- Minimizing waste and hazardous byproducts
- Developing eco-friendly processes by optimizing reagent use
This is a core principle in green chemistry, where stoichiometry ensures minimal environmental impact.
Tips to Master Stoichiometry
- Always balance your chemical equations first.
- Label units clearly and track them throughout your calculations.
- Use dimensional analysis for smooth unit conversions.
- Practice a variety of problems: mole-to-mole, mass-to-mass, volume-based